Low temperature fuel cells
The electrochemical process taking place in a fuel cell is simply electrolysis in reverse. Rather than passing a current through water (H2O) to split it into hydrogen (H2) and oxygen (O2), the fuel cell reacts H2 and O2 to produce current, with water as the harmless byproduct.
There are many different types of fuel cell that operate at a range of temperatures. For mobile transportation, low temperature designs are of the most interest, especially the Polymer Electrolyte Membrane (PEM) fuel cell. This offers the benefits of higher power density (1 kg/kW) and flexible performance at a lower cost per unit power (€/W) in a compact, lightweight package. The fundamental elements in the PEM fuel cell are the bipolar plates – see Figure 1.
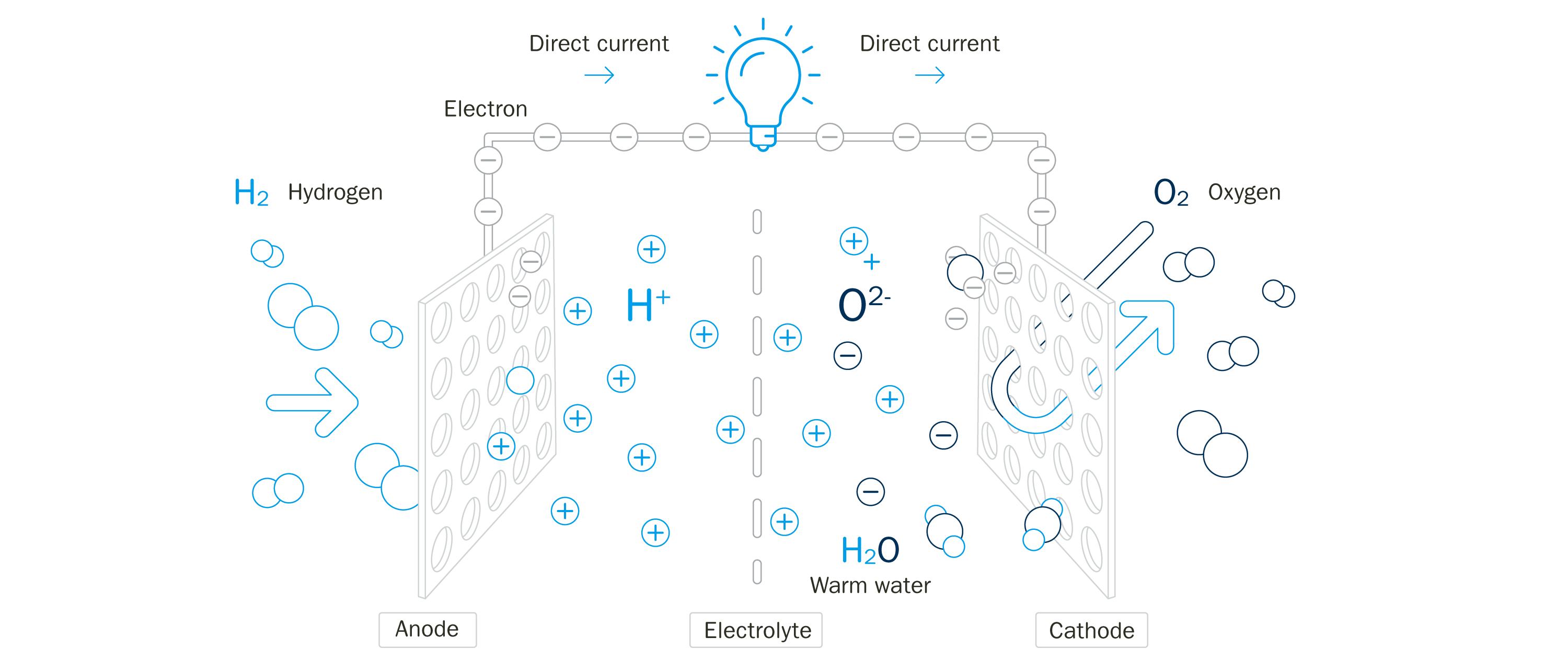
Figure 1. Bipolar plates are the fundamental elements in the PEM fuel cell.
The bipolar plates are responsible for transporting the H2 gas to the anode and the O2 gas to the cathode and bringing them into close proximity - separated only by the electrolyte membrane - so that they can react with each other. In a transportation fuel cell, the membrane is very thin, sometimes only around 20 microns. The plates also have to carry the current produced, as well as managing the water and heat that also result from the reaction.
Typically, an individual PEM fuel cell will produce around 1 V. Therefore, multiple cells are connected in series in a stack to provide the required voltage and power for the application. A stack will usually comprise 200 to 400 individual bipolar plates.

Figure 2. A complete fuel cell. A single stack will usually comprise 200 to 400 individual bipolar plates.
A demanding application for materials
A bipolar plate incorporates a complex pattern of channels machined or stamped into its surface as shown in Figure 3. This creates a “flow field” that enables the reactant gases to flow over the membrane electrode assembly (MEA). The plate may also feature internal channels for liquid cooling.
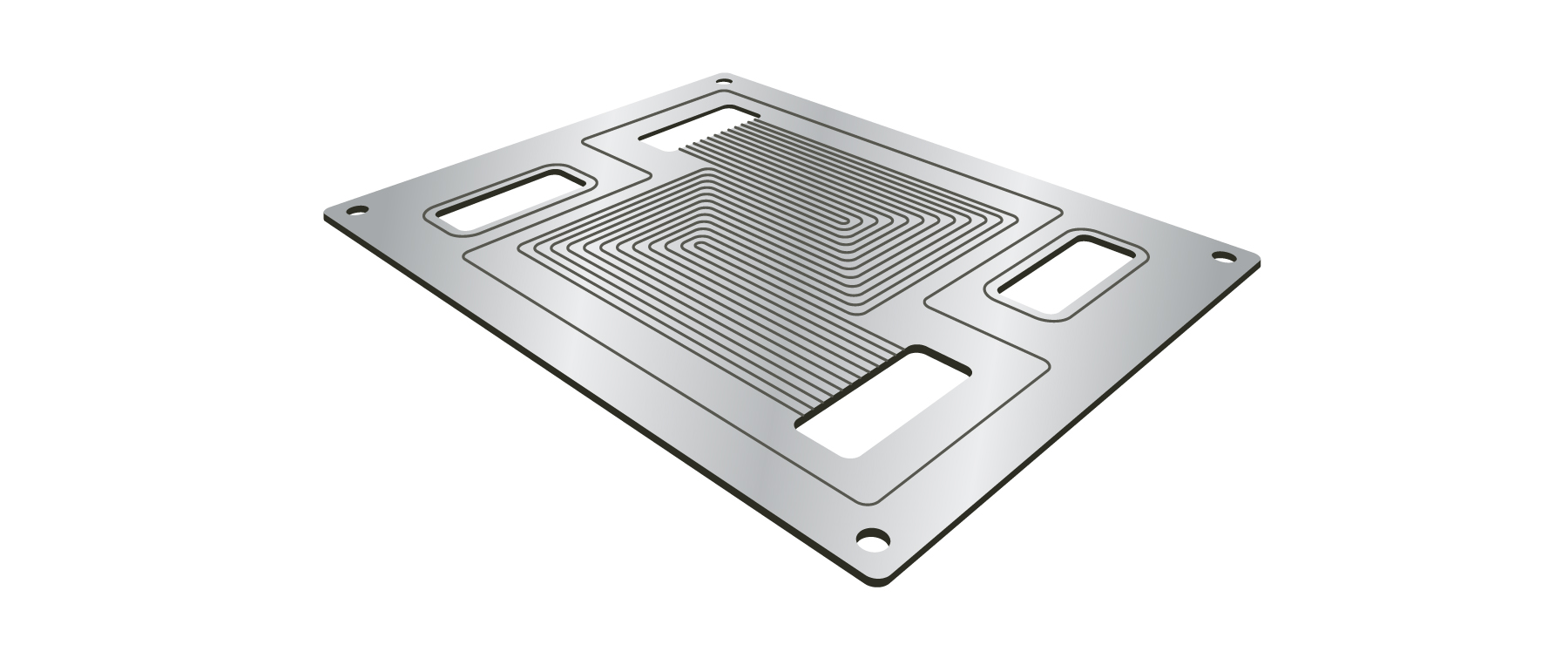
Figure 3. A bipolar plate features a complex pattern of channels formed into its surface.
The bipolar plates are very demanding on the materials used in their construction. This is because they must combine gas impermeability and tightness, good electrical and thermal conductivity and a high level of physical strength. Materials used include graphite, composites and metals.
Stainless steel for precision forming
CNC-machined graphite has been used successfully for bipolar plates in the early generations of PEM fuel cells with relatively low production runs. However, the high cost of graphite and the need for complex machining processes has prompted manufacturers to investigate alternative materials that are more cost-effective for mass production on a very large scale.
Stainless steel has emerged as a very strong contender, with Outokumpu’s own investigations indicating that Supra 316L (EN 1.4404) is the current state of the art. This molybdenum-alloyed austenitic grade offers the ideal combination of electrical conductivity, high corrosion resistance, excellent formability and high strength for the manufacture of bipolar plates.
While the natural corrosion resistance of stainless steel is very important, the side of the plate in contact with the electrolyte membrane still needs to be coated. This coating, which is only about 5 microns thick, is essential both to protect the base layer against the very corrosive electrolyte and to provide very high electrical conductivity. In this respect, stainless steel is at no disadvantage with rival metals such as titanium, which also requires a coating.
A key aspect where stainless steel offers a major advantage is in its excellent formability. This allows the precise and repeatable pressing of very thin bipolar plates that incorporate flow channels in their surface. To give an idea of how exacting this, the deepest channels are 0.1 mm, 0.075 mm is the current state of the art and we are already looking at new designs with channels of just 0.05 mm.
Stainless steel for tanks and piping
Stainless steel can also be used in other vital components in the complete fuel cell system, such as hydrogen storage and the piping that carries it to the fuel cell. Because of its ductility and toughness, stainless steel can support the design of a pressure-resistant tank as a material for the inner container or “Liner”. This enables safe and gas-tight storage of hydrogen.
There is always a concern when handling hydrogen under pressure due to the well-known phenomenon of hydrogen embrittlement of steel. This is where austenitic steel offers an advantage as its microstructure has a much greater resistance to hydrogen.
Ready to power the hydrogen future
Fuel cells can make an important contribution to creating a decarbonized transportation future. The challenge is in taking the technology that is already well proven in low volume applications to the mass market. Stainless steel offers significant potential to create cost-effective, durable and high performance bipolar plates suitable for very high volume production.
Stainless steel is also a superb material for sustainable solutions as it is long-lasting and 100% recyclable at the end of life, while the recycled content of Outokumpu’s own stainless steel is more than 90%. Furthermore, the production of the very thin materials required for fuel cells is part of the day-to-day business for our precision strip mill in Dahlerbrück, Germany.

